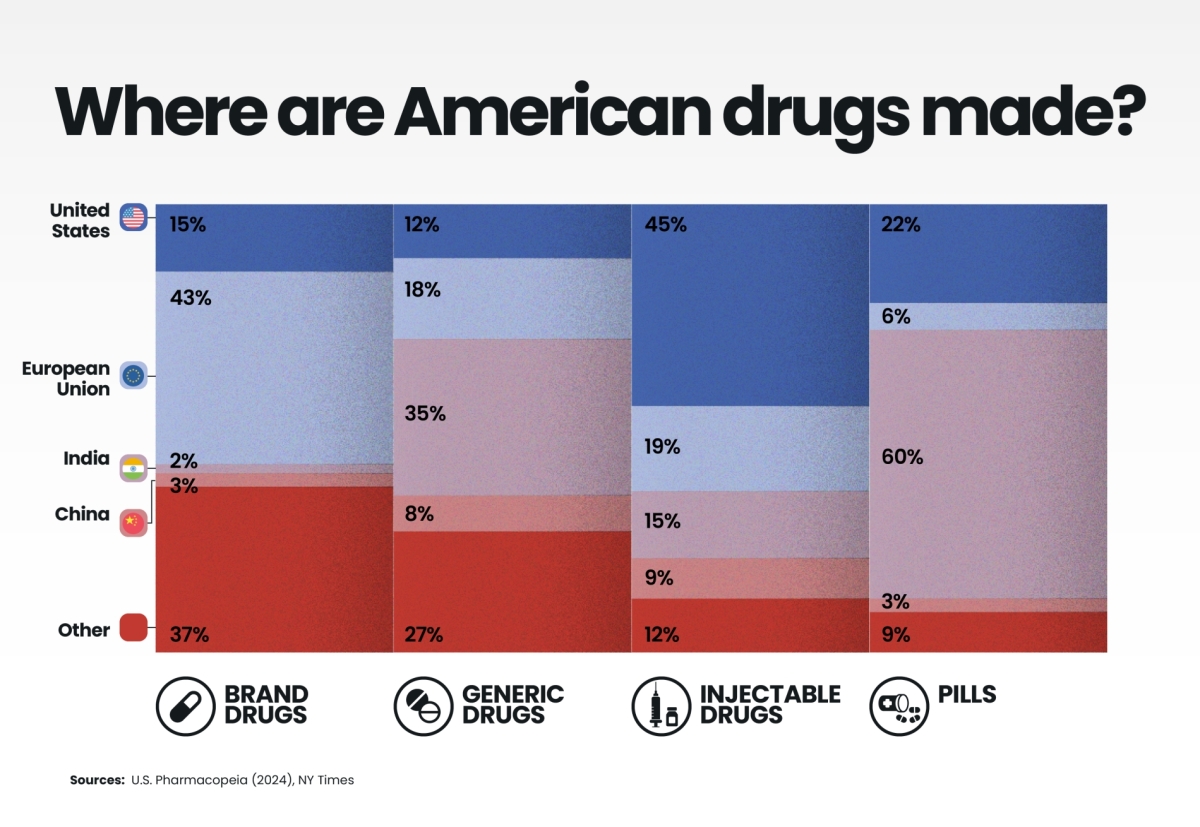
The geography of US drug manufacturing reveals a striking paradox: while America is home to the world’s leading pharmaceutical innovators, the production of its medicines—especially generics and pills—is largely outsourced abroad.
India, dubbed the “pharmacy of the world,” supplies over a third of America's generics and the majority of its oral tablets, while Europe dominates the production of branded drugs. Only in injectable medicines, which demand higher sterility and quality control, does the US retain significant domestic capacity.
This globalised structure offers clear economic advantages: lower costs, diversified supply, and access to specialised expertise. Yet it also exposes strategic vulnerabilities. Geopolitical tensions, export restrictions, or supply chain disruptions—such as those experienced during the COVID-19 pandemic—could threaten access to essential medicines. For US policymakers, this raises pressing questions about health security and the need to rebuild resilient domestic capacity.
Diversification also complicates FDA oversight. Every overseas facility producing for the US market must meet FDA Good Manufacturing Practice (GMP) standards, yet global dispersion stretches inspection capacity and delays approvals.